In the water/wastewater industry one group of reactions, which are very important, involve acids, bases and salts. Acids, bases and salts are commonly used for pH adjustment, flocculation, sedimentation, disinfection, corrosion control, water softening and taste, color and odour control.
When an acid, base or salt is added to water, the compound will split into two separate parts. When this occurs the compound is said to have disassociated. An acid is a compound, which will release hydrogen ions (H+) when it disassociates. The stronger the acid the more hydrogen ions the compound will release.
An example of an acid hydrochloric acid:
HCl ~~ H+ + Cl-
Hydrorochloric Acid ~~ Hydrogen ion + Chloride ion
When a base is added to water and disassociates it will release hydroxyl ions (OH). The stronger the base the more hydroxyl ions will be released. This is illustrated in the equation below.
NaOH ~~ Na+ + OH-
Sodium Chloride ~~ Sodium ion + Chloride ion
A salt will not release hydrogen or hydroxyl ions when it dissociates. A good example is common salt, sodium chloride;
NaCl ~~ Na+ + Cl-
Sodium Chloride ~~ Sodium ion + Chloride ion
PH Scale
The pH scale is a method used to measure the strength of an acidic or basic solution (basic solutions may also be referred to as alkaline solutions). The scale ranges from 0-14 with acids having a pH of less than 7, and bases a pH greater than 7. A solution, which is neither acidic nor basic, is called a neutral solution and has a pH of 7. Each increase in 1 in the pH scale indicates a solution, which is 10 times more basic. For example a liquid with a pH of 8 is 10 times more basic than a liquid with a pH of 7.
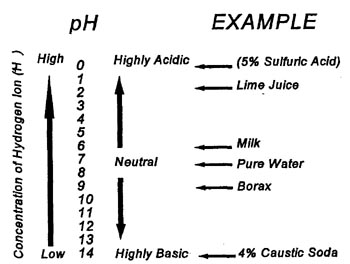
When acid and base solutions are combined a salt will form and the hydrogen (H+) and the hydroxyl (OH-) ions will combine to form water (H2O). If the acid and base solutions are approximately the same strength (i.e. the same amount of hydrogen and hydroxyl ion concentrations) the solutions will neutralize. This is an important reaction in the water/wastewater treatment industry.
Acidity and alkalinity are terms used to describe a waters ability to buffer a base or an acid respectively. Acidity and alkalinity are measured in milligrams per litre equivalent amounts of calcium carbonate.
Solutions
A solution is made up of two or more different parts. One component is known as the solvent, which will dissolve the other components(s) which are called the solute(s). For example when sugar is dissolved in water, the sugar is the solute and the water is the solvent. The concentration of a solution refers to the amount of solute, which is dissolved in the solvent. The greater the amount of solute the higher the concentration. There are several methods for measuring the concentration of a solution. These include molarity (M), normality (N), and calculating the mass of a solute, as a percent of the total mass of the solution is the most common coagulant, many other chemicals are used.
Chlorine (Cl)
Chlorine is the most common method of disinfections used in Ontario. Chlorine is available as a solution in water (sodium hypochlorite), powder (calcium hypochlorite) or gas. The liquid is similar to a strong “Javex” solution and is relatively safe to handle. Chlorine gas, on the other hand, is extremely dangerous to handle, a severe respiratory irritant, which may result in death, and is flammable and explosive.
Chlorine gas is a greenish/yellow color. It has a penetrating odour. It is heavier than air and thus will concentrate in low lying enclosed areas.
Chlorine gas is supplied in pressurized metal cylinders. These cylinders must be handled with special care. Because of the pressure within the cylinders, chlorine is in liquid form. As the chlorine is depressurized into a gas its volume increases 450 times. Before working with chlorine gas, operators must ensure that they are aware of the mechanical procedures, safety procedures and how to use emergency leak stoppage equipment.
Leaks of chlorine gas can be detected by using commercial, which when passed below the leak produces a white smoke. To work on chlorine gas leak an operator must wear a self-contained breathing apparatus.
Chlorine, in both solution and gas forms is an effective low cost method of disinfections. When using chlorine, it is important to ask how much chlorine is required and how long the chlorine should be in contact with the water. The amount of chlorine used is called the dosage. The time required for disinfections to occur is called the contact time. When chlorine is first introduced to water it will combine with organic and inorganic compounds. The chlorine, which is required to combine with these compounds, is called the chlorine demand. Some of the chlorine will react with ammonia to create what is often called combined chlorine. This form of chlorine has good disinfecting properties. Chlorine, which does not combine with other compounds, is known as free chlorine. The sum of the combined chlorine compounds and free chlorine is known as the chlorine residual.
Chlorine dosage=chlorine demand + chlorine residual
Mg/L mg/L mg/L
Chlorine residual = combined chlorine + free chlorine
Mg/L mg/L mg/L
Free chlorine is a more effective disinfectant than combined chlorine, but is not as stable or as long lasting. An advantage of chlorine over other disinfections techniques is its ability to disinfect long after it was applied. This is important for ensuring that water remains safe in a distribution system or water storage tank.
Alternative methods of disinfections are becoming ever more common as a result of concerns regarding the long term effects of chlorine compounds (trihalomethanes) on humans and the environment. Trihalomethanes are produced when chlorine reacts organic material. These alternatives include ozone, ultra violet light as well as alternative chemicals such as chlorine dioxide.
Color
Many surface waters, particularly those originating from waters with decaying or organic debris, will have color, which is unacceptable to domestic customers and to some industrial processes. Color is one of the more difficult problems to handle in the treatment of water. Color compounds may also be precursors to trihalomethane formation.
Hardness
Hard waters are those that, as a result of high dissolved mineral salts, require considerable amount of soap to produce a foam or lather. Hard water will also leave a scale in hot water heaters and boilers. Hardness is primarily caused by the presence of dissolved calcium and magnesium salts. Hardness is normally found in waters running through or over limestone. Hardness is expressed in milligrams per litre as calcium carbonate (mg/L as CaCo3). Hardness can be removed using chemicals in treatment plants or using ion exchange water softeners in the home.
Nitrogen
Nitrogen is a vital element for the life processes of living organisms. Any discussion of nitrogen in this regard is very complex because of the very large numbers of compounds, both organic and inorganic, associated with nitrogen. Nitrogen is an important consideration for wastewater effluent since it acts as a nutrient, which stimulates plant growth. In terms of drinking water, nitrites are known to be toxic, particularly to infants. In addition the presence of nitrogen compounds is often an indication of pollution.
Oxygen (O)
The amount of free oxygen in water is known dissolved oxygen. The total amount of oxygen, which can be dissolved in water, is dependant upon temperature. The level of oxygen in water will determine what types of organisms can survive. The release of untreated or partially treated sewage may lower a stream’s dissolved oxygen level, thereby making it uninhabitable to many species of plants, fish and other animals. In the absence of oxygen a body of water is said to be septic. From a treatment perspective certain types of wastewater treatment processes can only occur in the presence or absence of oxygen. Thus maintaining the correct dissolved oxygen content is very important in many processes within a wastewater treatment plant.
The effect wastewater has upon a stream is often estimated based upon the Biochemical Oxygen Demand (BOD) of the wastewater. BOD is the amount of oxygen required by bacteria while stabilizing decomposable organic matter under aerobic conditions. The BOD test is used to determine the organic strength (i.e. amount of organic pollution) of wastewater. The higher the BOD, (expressed as mg/L), the greater the amount of organic material.
Phosphorus (P)
Phosphorus and phosphorus compounds are important in both water treatment and wastewater treatment. In industrial water treatment, phosphates are applied to water to control scaling in boilers. Phosphorus is also a requirement for plant and bacterial growth. Therefore, some phosphorus is necessary in biological wastewater treatment processes (i.e. activated sludge) to ensure that there are sufficient nutrients for the microorganisms to remain viable. On the other hand, excessive phosphorus discharged into a receiving stream promotes plant growth leading to high levels of algae and other plant materials in the water. Wastewater treatment plants which discharge to the Lower Great Lakes area re required to remove excess phosphorus from their effluents.
Solids
The determination of solids concentrations in water is quick and simple. These measurements thus provide a very quick indication of water quality. There are numerous types of solids that are analyzed, including:
Turbidity
The term turbidity is applied is applied to waters containing suspended matter which interferes with the passage of light. It can be caused by a wide variety of suspended materials ranging in size from colloidal to coarse particles. Collides are very small particles suspended in water which cannot be removed through sedimentation or filtration without the use of chemicals. Turbidity can result from erosion or disturbance of river bottoms, and material originating from domestic and industrial wastewaters. Turbidity itself may not be dangerous to human health but may provide an indication of pathogenic or other harmful material.
From a public perspective turbidity has two main effects. The first is aesthetic; a customer of drinking water expects to receive water with no noticeable cloudiness. Secondly, turbidity allows harmful microorganisms to become enveloped within solids and there fore protect themselves from disinfections. Turbidity removal normally requires both physical and chemical processes.